Lifescan Announces Future Innovation at EASD: The OneTouch Verio Reflect™ Blood Glucose Meter featuring Blood Sugar Mentor™
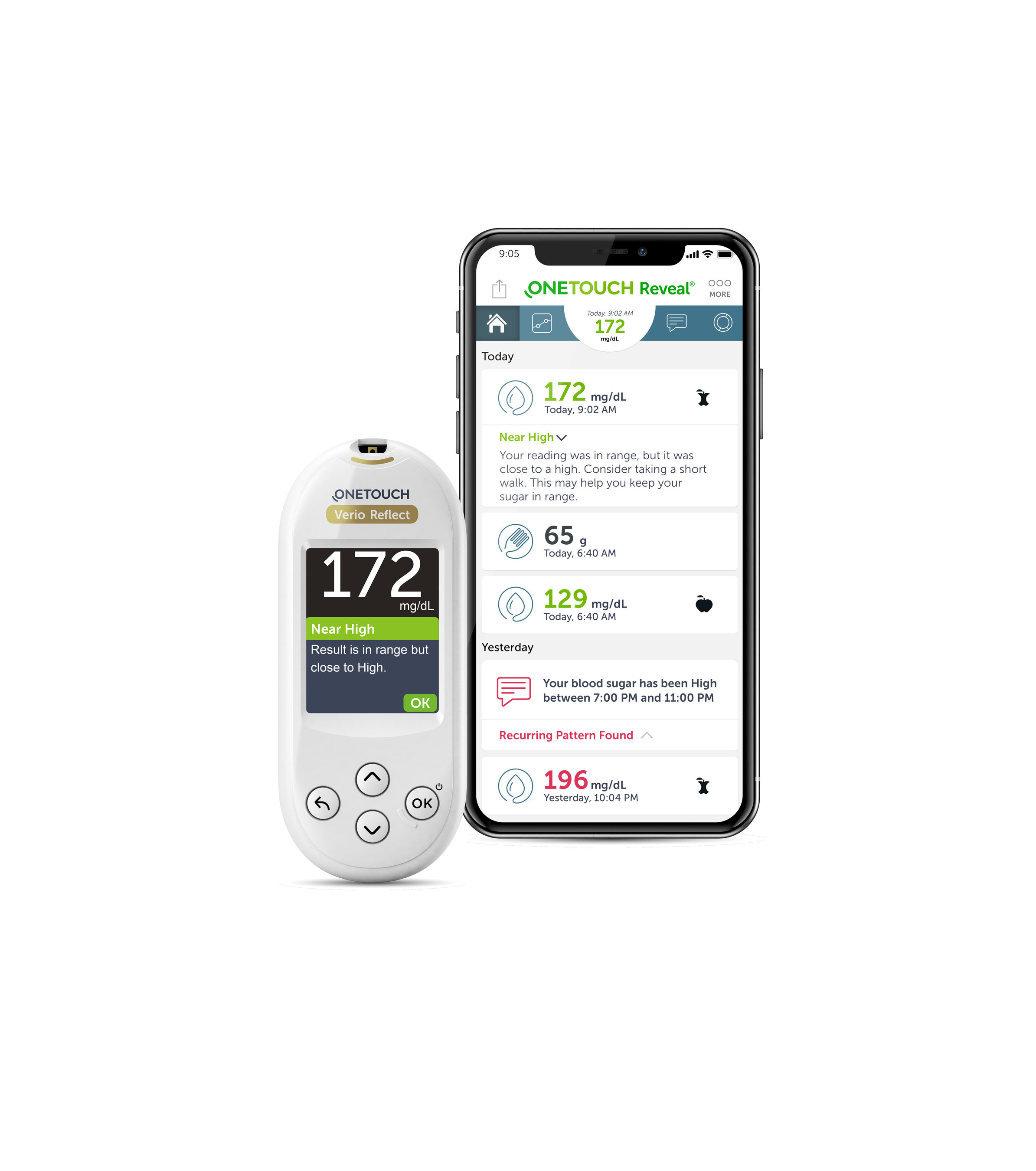
BERLIN, Oct. 4, 2018 /PRNewswire/ — Lifescan, Inc., a world leader in blood glucose monitoring, revealed its latest innovation—the OneTouch Verio Reflect™ blood glucose meter—as part of a symposium on the future of connected self-monitoring of blood glucose at the European Association for the Study of Diabetes (EASD) 2018 annual meeting today.
The OneTouch Verio Reflect™ system features the Blood Sugar Mentor™1, which is designed to analyze patterns, inform patients of blood glucose trends, and provide them with personalized guidance which could help detect when blood sugar levels are rising above or falling below a desired range. It also features a ColorSure® Dynamic Range Indicator (ColorSure® PLUS) that is an expansion of ColorSure® technology from the OneTouch® brand. ColorSure® PLUS technology is designed to help patients understand when glucose results are nearing highs or lows. The OneTouch Verio Reflect™ meter will also unlock user-inspired features in the OneTouch Reveal® mobile app, which continues to be one of the top downloaded diabetes apps globally2.
“The OneTouch Verio Reflect™ system delivers real-time, personalized guidance and encouragement based on a patient’s blood glucose results. The new system can be customized to individual patients and their needs over time,” says David DeJonghe, Head of Portfolio Strategy, Lifescan, Inc., who announced plans for the new product during the symposium. “Lifescan has a rich heritage of innovation in blood glucose monitoring. We will continue to innovate for patients and accelerate progress with solutions such as OneTouch Verio Reflect™ and our diabetes management app, OneTouch Reveal®.”
The new system is the culmination of a Lifescan-sponsored symposium where top international experts in diabetes care declared the continuing importance of self-monitoring of blood glucose (SMBG) and the possibilities of connected SMBG systems which use smartphones as an enabler and acknowledged the still unmet need for consistent patient support and interaction.
“Many people living with type 2 diabetes really don’t want visibility of their diabetes, rather they want discretion and the ability to choose, so wearing a device full-time would not be their preferred solution. Blood glucose monitors remain the most accurate means of testing at the lowest cost,” said Katharine Barnard, Ph.D., visiting professor of Health Psychology at both Bournemouth University and the University of Southampton and UK-based Chartered Health Psychologist who specializes in the psychosocial impact and management of diabetes. “The development of next-generation connected SMBG tools will minimize the time people have to think about their diabetes tasks while helping their self-management with reduced diabetes burden. That will be a real win.”
The symposium, chaired by Steven Edelman, M.D., clinical professor of medicine at the Center for Metabolic Research and founder of Taking Control of Your Diabetes (TCOYD), a nonprofit diabetes education organization, also announced the completion of a consensus statement entitled “Bridging to the Future: SMBG is Not Dead, it’s Under-Appreciated.” This statement is the conclusion of multiple sessions held with various diabetes leaders and stakeholders during the past year on the continued importance of SMBG and opportunities for expanded potential. The authors have plans to publish the statement in a peer-reviewed journal later this year.
The OneTouch Verio Reflect™ meter is awaiting CE marking certification with commercial launch in France anticipated for Q4 2018 with other EMEA markets following in 2019. The product has also been submitted to the U.S. Food and Drug Administration (FDA) for 510(k) clearance, and to Health Canada for a Medical Device Licence. In addition, the OneTouch Ultra Plus Reflect™ meter – a blood glucose meter with the same features as the OneTouch Verio Reflect™ meter that utilizes OneTouch Ultra Plus® test strips in place of the OneTouch Verio® test strips – is also awaiting CE marking certification with commercial launch in Germany and Belgium anticipated for Q4 2018, and other EMEA markets following in 2019.
The OneTouch Reveal® mobile app is available as a free download for Apple® devices on the App Store® and Android™ devices on Google Play™ in 20 countries globally, including Argentina, Algeria, Austria, Belgium, Brazil, Canada, Colombia, Chile, France, Germany, India, Italy, Ireland, Mexico, Portugal, Qatar, Spain, the United Arab Emirates, the United Kingdom, and the United States.
About Lifescan, Inc.
Lifescan, Inc. is a world leader in blood glucose monitoring – globally more than 20 million people depend on OneTouch® brand products to help them manage their diabetes, and the OneTouch Verio® platform has demonstrated seven years of proven accuracy across more than 70,000 clinical data points3. In the U.S. Lifescan, Inc. is the leading blood glucose monitoring company and OneTouch® brand products are recommended by more endocrinologists, primary care physicians, and pharmacists than any other brand. The new OneTouch Verio Reflect® System and the OneTouch Verio Flex® meter feature ColorSure® technology that instantly shows when patients’ blood glucose results are in or out of range, and built-in Bluetooth®i Smart Technology enables both meters to connect wirelessly and sync seamlessly with the OneTouch Reveal® mobile app, which continues to be one of the top 10 most downloaded diabetes management apps globally4. Both the OneTouch Verio® Reflect system and the OneTouch Verio Flex® meter work with the OneTouch Reveal® mobile app to help people with diabetes and their healthcare teams harness the power of information to take a step forward in managing their diabetes. For more information, visit: www.OneTouch.com.
1 Blood Sugar Mentor™ refers to the Pattern Messages, Mentor Messages or Awards Messages that may appear with Blood Glucose results
2 R2G Data, August 2018
3 Setford, et al. Seven-year surveillance of the clinical performance of a blood glucose test strip product. Journal of Diabetes Science and Technology (2017) 1-85
4 R2G Data, August 2018
i The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG, Inc., and any use of such marks by Lifescan Scotland Ltd. is under license. Other trademarks and trade names are those of their respective owners.
SOURCE Lifescan, Inc.





